ELISA Assay Development & ELISA Kit Manufacturing Services
This service provides end-to-end development of ELISA assays for target substances using your existing antigens and/or antibodies.
We also offer contract manufacturing of ELISA kits on a small scale.
The typical development period for assay establishment is approximately three months.
If you do not have antigens or antibodies available, please feel free to consult with us—we offer flexible support.
Please note that depending on the characteristics of the antigens, antibodies, or target substances, assay development may not be feasible. In such cases, we will discuss alternative options with you separately.

Items to Be Confirmed
・Target substance
・Measurement range
・Sensitivity: Limit of Detection (LOD)
・Accuracy
・Sample type
*For measurement range, sensitivity, and accuracy, we will conduct preliminary studies in advance to meet your desired specifications.
Measurement Results
- ・Measurement results are expressed in ng/mL or pg/mL and quantitatively calculated based on a standard curve.
- ・Abnormal values or values exceeding reference limits are determined according to predefined criteria.
Quality Control & Assurance
-
Quality Control:
◦ All reagents and components undergo quality testing prior to shipment, and only those that pass are released.
◦ Performance testing is conducted for each lot, and products are provided based on verified results. -
Quality Assurance:
◦ Consistent quality is ensured through standardized testing protocols and documented verification processes.
◦ Lot-to-lot consistency is maintained and monitored to ensure reliable assay performance.
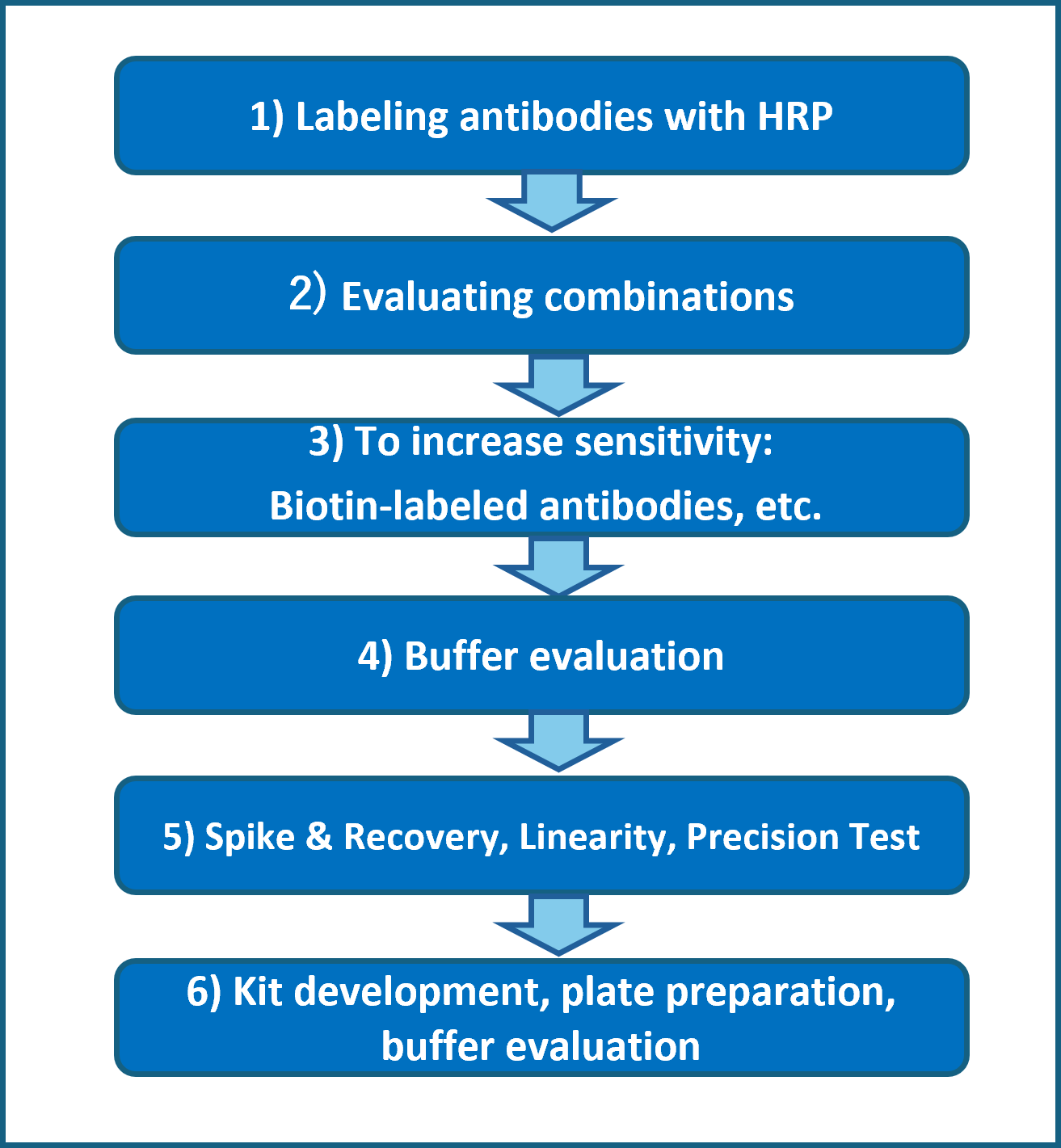
ELISA Kit Development Flow (Example)